At SYNTHIA, we believe that meaningful innovation begins with solid foundations.
That is why the architecture of our project has been carefully designed to match the scale of our ambition: to drive responsible, validated use of synthetic data in healthcare across Europe.
From the outset, SYNTHIA has been structured to foster deep collaboration across disciplines, sectors, and borders. With 32 partners, our consortium reflects the complexity and promise of the synthetic data ecosystem we aim to transform.
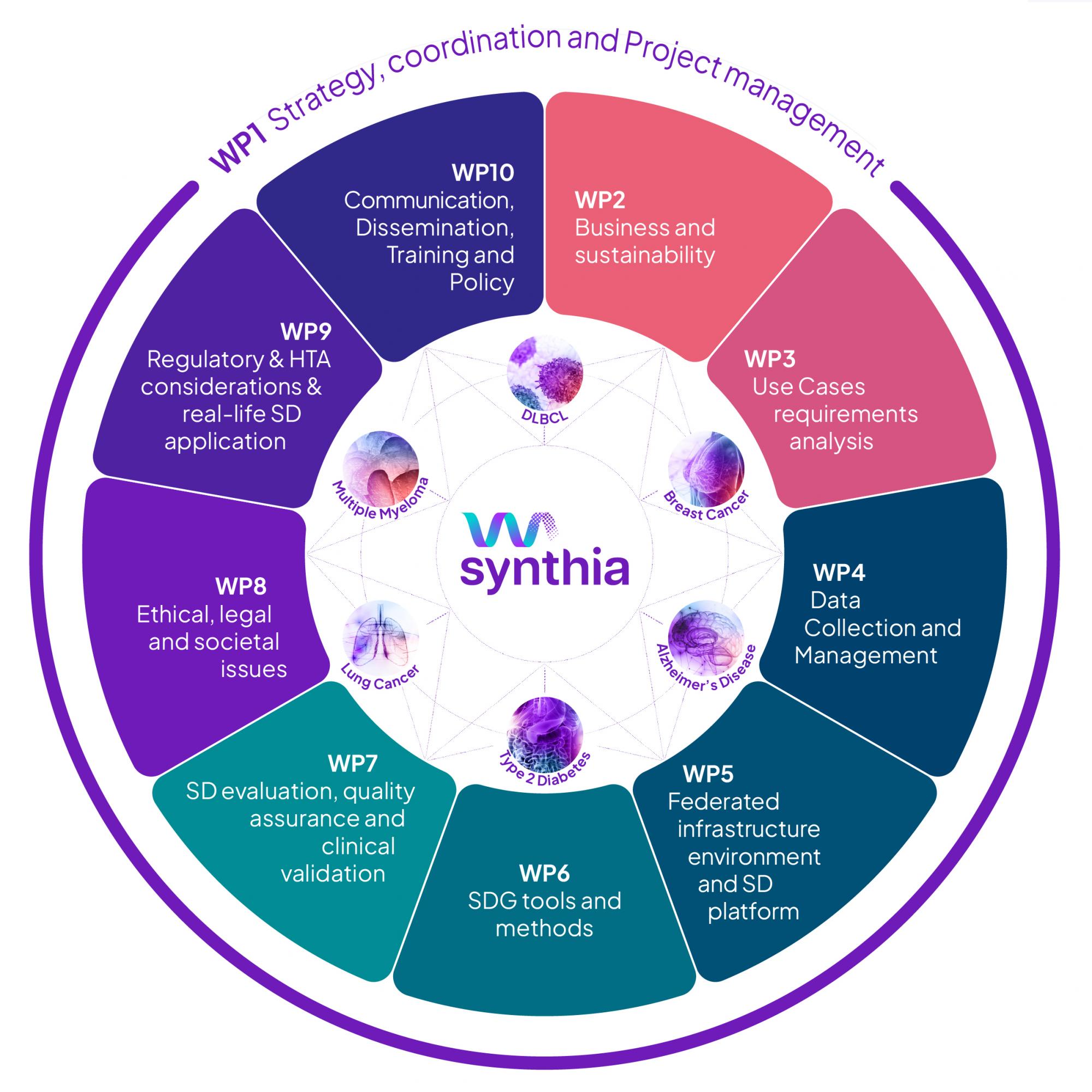
To navigate this complexity and ensure progress is strategic, transparent, and measurable, SYNTHIA is built around a series of interconnected Work Packages (WPs), each dedicated to a vital domain of the project, and grounded in six Use Cases (UCs) that represent real-world clinical challenges.
This structure allows us to align scientific discovery, technical development, regulatory dialogue, and societal engagement around a shared mission, while remaining agile and responsive to emerging needs.
WP1 Strategy, Coordination and Project Management: Ensures smooth, efficient management across the consortium, overseeing governance, reporting, and communication.
WP2 Business and Sustainability Models: Explores economic models and value frameworks to sustain SYNTHIA’s impact beyond the project duration.
WP3 Use Case Requirements and Integration: Gathers clinical and technical needs to define what synthetic data must achieve in each of the six use cases.
WP4 Data Collection and Management: Coordinates access to high-quality real-world data, ensuring ethical compliance, harmonisation, and readiness for synthetic generation.
WP5 Federated Infrastructure Design and Implementation: Builds a secure, interoperable platform where synthetic data generation tools, workflows, and datasets can be accessed and validated.
WP6 Synthetic Data Generation (SDG) Tools and Methods: Develops and refines state-of-the-art tools—ranging from statistical models to machine learning approaches—for generating high-quality synthetic data.
WP7 Evaluation, Validation and Quality Assurance: Assesses the fidelity, utility, and fairness of synthetic datasets across technical, clinical, and ethical dimensions.
WP8 Ethical, Legal and Societal Impact (ELSI): Addresses the legal and societal implications of synthetic data, guiding responsible innovation in line with EU regulations and values.
WP9 Regulatory and HTA Engagement: Interfaces with regulators, payers, and health technology assessment bodies to support real-world adoption and trust.
WP10 - Communication, Dissemination, Training and Policy: Leads stakeholder engagement, knowledge sharing, policy alignment, and capacity-building throughout the project.